 Photons -
How We
See
Photons -
How We
See Photons -
How We
See
Photons -
How We
See Before saying more
about photons, it is prudent to say
what physics
tries to do. Physics tries only to describe how objects behave, what they do in response
forces. Physics does not try to say why things behave that way, nor
what mechanism makes that behavior happen. Mechanism
would be nice, but no one knows what "really" happens.
Before saying more
about photons, it is prudent to say
what physics
tries to do. Physics tries only to describe how objects behave, what they do in response
forces. Physics does not try to say why things behave that way, nor
what mechanism makes that behavior happen. Mechanism
would be nice, but no one knows what "really" happens. | In the latter half of the
nineteenth century physicists saw objects as
collections of particles (atoms) and forces as waves. We can see both
in a shoreline. But what is light? a particle or a wave? |
 |
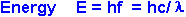 where h, Planck's constant, has the value of 6.6 x 10-34
J.s, and c is the speed of light in a vacuum, with a value of 3.0 x 108
m.s-1. When the light interacts with
matter, energy is absorbed only as discrete packets and all
the energy of the packet is transferred. Thus, if the energy of a
photon is sufficient to excite an electron, it will do so, but if it it
insufficient, it will not.
where h, Planck's constant, has the value of 6.6 x 10-34
J.s, and c is the speed of light in a vacuum, with a value of 3.0 x 108
m.s-1. When the light interacts with
matter, energy is absorbed only as discrete packets and all
the energy of the packet is transferred. Thus, if the energy of a
photon is sufficient to excite an electron, it will do so, but if it it
insufficient, it will not. The
most important thing to know about photons is when they meet
electrons. When an electron "absorbs" a photon, the elctron gets a
little kick in its energy. If this electron is in an atom, it jumps
from its present orbit to the next outer orbit. An electron can also
lose energy; then it emits a phton of the same frequency as the one
whose energy it needed to jump to that outer orbit. It is these emited
photons that we see as light from the sun.
The
most important thing to know about photons is when they meet
electrons. When an electron "absorbs" a photon, the elctron gets a
little kick in its energy. If this electron is in an atom, it jumps
from its present orbit to the next outer orbit. An electron can also
lose energy; then it emits a phton of the same frequency as the one
whose energy it needed to jump to that outer orbit. It is these emited
photons that we see as light from the sun.|
The photosensitive
molecule
involved in vision is called rhodopsin, (also
known as visual purple) which consists of a large protein
(having a molecular weight of around 38,000) called opsin,
joined to 11-cis-retinal via a protonated Schiff base on one of
its lysine side-chains.
|
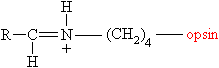 |