|
Victorian
physics treated energy, heat, light, space, gravity, and other
phenomena as
continuous. If one had a certain amount of space or light, one could as
easily have half that amount. It was infinitiely divisible into finer
units. Planck made the astonishing discovery that a certain calculation
could only be done if radiant heat was not continuous but came in
little
bundles. He called them "quanta." Einstein--exactly a century ago--said
that light, too, came in little bundles. We call them photons today.
There was a bit of a problem. Light is also seen to behave like a wave
in some experiments. For instance, white light splits into colors in a
prism. The final situation seems to be that each photon is a little
"wavelet" with intensity varying a its little space. That intensity can
be described with the same sort of equation that can describe water
ripples where a rock has fallen through. Ripples further from the
center are smaller. In the same way, the ripples of a wavelet fall off
in intensity at distances from the center of the photon. They fall off
much more rapidly so that the photon appears to be pretty much at one
place.
About the same time, other particles were discovered; notably
electrons, neutrons and protons. Everything tangible that we know of is
made of these as they are grouped into atoms. An atom is a nucleus of
neutrons and protons surrounded by a cloud of electrons. The electrons
are gathered to the nucleus because they have negative electric charge
and the protons in the nucleus have positive charge. There are always
more neutrons than protons and these neutrons play a role in preventing
the protons from themselves flying apart and scattering the atom.
Further experiments show that all particles are capable of wave-like
behavior. Schroedinger developed his Wave Function to describe the
intensity of any given particle at each point surrounding its putative
center. Thus all particles--all of physical existence--came to be
understood as instances of quanta.
The "Wave-particle Duality" notion expresses the situation by stating that every particle, not just photons, can be treated as either a wave or a particle. In some experiments the objects behaves like a particle. For instance, it bounces. In other experiments it behaves like a wave. Usually this is observed as the particle "interfering" with itself.

We can see interference of photons in most roadway puddles, as beautifully explained by Dinesh O. Shah.
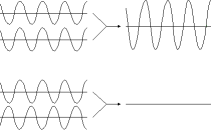
Interference occurs when two light waves arrive at the same point. If they are both at their highest, the values add and the light at that point is brighter. this is in the top half of the diagram. But if one is high and the other low, they counteract each other and there is no light. If the light is a single color, the result will be dark or that color, as shown in the bottom half of the diagram
In the puddle the incoming sunlight is all colors and the oil film varies in thickness. This happens for all colors, each with its own wavelength. The wave bouncing off the top of the oil film has a different travel time than that from the back, so the peaks of the waves have diferen relations at each point. Some colors cancle out and others are enhanced.
|